New sterilization technology based on plasma was patented in 1987 and marketed in the United States in 1993. Gas plasmas have been referred to as the fourth state of matter (i.e., liquids, solids, gases, and gas plasmas). Gas plasmas are generated in an enclosed chamber under a deep vacuum using radiofrequency or microwave energy to excite the gas molecules and produce charged particles, many of which are in the form of free radicals. A free radical is an atom with an unpaired electron and is a highly reactive species.
The proposed mechanism of action of this device is the production of free radicals within a plasma field that is capable of interacting with essential cell components (e.g., enzymes, nucleic acids) and thereby disrupt the metabolism of microorganisms. The type of seed gas used and the depth of the vacuum are two important variables that can determine the effectiveness of this process.
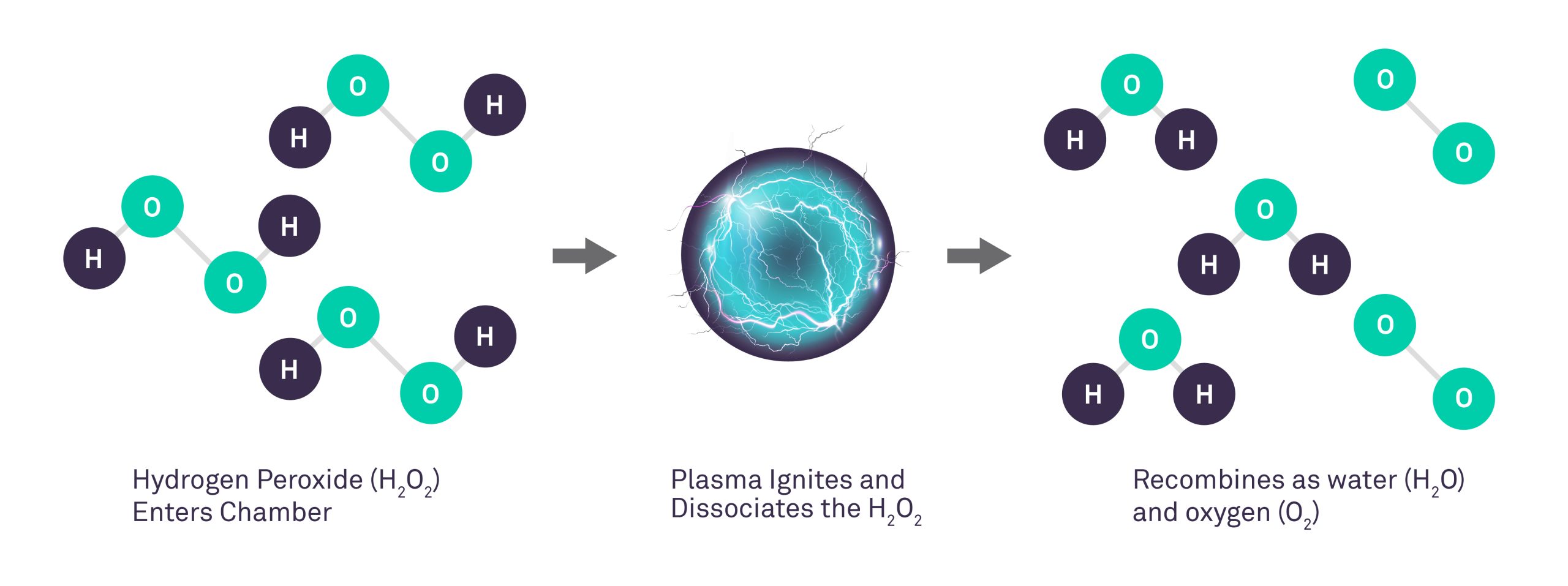
In the late 1980s, the first hydrogen peroxide gas plasma system for sterilization of medical and surgical devices was field-tested. According to the manufacturer, the sterilization chamber is evacuated and the hydrogen peroxide solution is injected from a cassette and is vaporized in the sterilization chamber to a concentration of 6 mg/l. The hydrogen peroxide vapor diffuses through the chamber (50 minutes), exposes all surfaces of the load to the sterilant, and initiates the inactivation of microorganisms. An electrical field created by a radio frequency is applied to the chamber to create a gas plasma.
Microbicidal free radicals (e.g., hydroxyl and hydroperoxyl) are generated in the plasma. The excess gas is removed and in the final stage (i.e., vent) of the process, the sterilization chamber is returned to atmospheric pressure by the introduction of high-efficiency filtered air. The by-products of the cycle (e.g., water vapor, oxygen) are nontoxic and eliminate the need for aeration. Thus, the sterilized materials can be handled safely, either for immediate use or storage. The process operates in the range of 37-44°C and has a cycle time of 75 minutes. If any moisture is present on the objects the vacuum will not be achieved and the cycle aborts.
Microbicidal Activity
This process has the ability to inactivate a broad range of microorganisms, including resistant bacterial spores. Studies have been conducted against vegetative bacteria (including mycobacteria), yeasts, fungi, viruses, and bacterial spores. Like all sterilization processes, the effectiveness can be altered by lumen length, lumen diameter, inorganic salts, and organic materials.
Uses
Materials and devices that cannot tolerate high temperatures and humidity, such as some plastics, electrical devices, and corrosion-susceptible metal alloys, can be sterilized by hydrogen peroxide gas plasma. This method has been compatible with most (>95%) medical devices and materials tested.
Friday, Nov 27, 2020
Content source:
Please Check out file at the following link
Plasma Biological Indicator 10^6 (RRS 14-23110)
Double self-adhesive Plasma label (RRS 14-13110)